Immunotherapy for Colon Cancer
Colon cancer or colorectal cancer (CRC) is a serious cancer type with high incidence and mortality rates in developed countries. Colon Cancer develops in the tissues of rectum or colon. It spreads to the liver, lungs, ovaries, and other parts of the gastrointestinal system, making it one of the most dangerous malignancies.
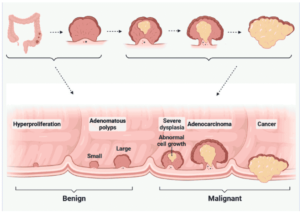
Developed countries have high incidence and mortality rates for colon cancer or colorectal cancer (CRC). Colon cancer ranks third in the United States among cancers diagnosed in both men and women. In the United States, colorectal cancer is the third most diagnosed cancer in both men and women. Colon cancer and rectal cancer are often grouped together because they have many common features.
Researchers grouped rectal cancer, colorectal cancer, and other types of cancers related to colon cancer under the title of colon cancer for analysis. The precursor of colon cancer is polyps that turn into cancerous cells over time. Polyps, which turn into cancerous cells over time, are the precursor of colon cancer. Colonoscopy is the most widely accepted standard for detecting these polyps and screening for colon cancer. Colonoscopy is widely accepted as the standard for detecting polyps and screening for colon cancer. And immunotherapy for colon cancer also accept for many reasons. Here it will discuss in detail.
Global statistics of Colon Cancer
According to estimates from GLOBOCAN 2020, researchers predict that there will be 1.15 million new cases of colon cancer worldwide in 2020. These numbers are expected to increase to 1.92 million in 2040. In 2020, experts anticipate diagnosing 1.93 million new Colorectal cancer (CRC) cases, with 0.94 million individuals projected to die from CRC. By 2040, the global healthcare community expects to see 3.2 million additional CRC cases worldwide. Chronic inflammation with oxidative cellular injury is believed to play a significant role in the development of colon cancer, which can be caused by various factors. However, two of the most significant factors are personal history and family history, in addition to other risk factors like genetic vulnerability involving somatic mutations of the tumor suppressor gene adenomatosis polyposis coli (APC).
Reasons that cause Colon Cancer
Molecular pathophysiology epidemiology (MPE)
is a multidisciplinary and interdisciplinary study field investigating the relationship between risk factors (microbiome, germline genetics, dietary pattern, lifestyle, and other factors) and genetic markers, innovation, incidence, and disease progression. It has been the dominant approach for performing observational cancer research since its inception. Various genetic disorders, such as BRAF mutations, microsatellite instability (MSI), KRAS mutations, and PIK3CA mutations, have been associated with the development of colorectal cancer (CRC). CRC is linked to microsatellite destabilization (MSI), BRAF alterations, PIK3CA mutants, genetic changes in KRAS, and the elevated CpG island methylator phenotype. Numerous studies conducted in recent years have revealed that the dysregulation of up to 500 gene products, not just heritable mutations, is responsible for the majority of malignancies.
Most risk factors associated with colorectal cancer including
- grilled meat
- fried meals
- saturated fatty acids
- psychological
- physical stress
- environmental contaminants
have been shown to activate this transcription factor. Previous research has demonstrated that smoking and obesity are both major causes of colorectal cancer. The way food is prepared and consumed can also influence colorectal cancer risk. People who consume high amounts of calcium are less likely to develop colorectal cancer, as calcium plays a role in T cell activation, an important part of the immune system’s defense against colorectal cancer. Conversely, alcohol consumption has been linked to an increased risk of colorectal cancer.
Dietary supplements for colon cancer prevention
Nutraceuticals and their active metabolites have shown effectiveness against a variety of colon cancer cell lines. In vitro studies have indicated that antioxidant fruits like black raspberry, strawberry, and grape seeds can help prevent intestinal cancers.
Garlic, with its diverse nutritional profile and components such as organosulfur and S-allylcysteine, is crucial for preventing colon cancer in clinical models. Experimental tests have shown that garlic extract can reduce the formation of cancer cells in the early stages, although it may be ineffective in later stages. However, there is not enough scientific evidence to support its effectiveness for cancer prevention. Furthermore, garlic extract can be harmful as it may cause hemolysis and worsen a patient’s anemia.
Fenugreek is known to contain a high concentration of the steroidal saponin diosgenin.
Honey (eugenol)
Eugenol, a honey-derived component found naturally in cinnamon, clove oil, citrus, Flos Magnolia, and balm plant extracts, has potential medicinal uses in treating a variety of chronic diseases. Researchers have discovered that eugenol can increase apoptosis in colon cancer cells, making it a promising therapy for this type of cancer. It stimulates sub-G1 cell division, triggering apoptosis in a time-dependent manner. Eugenol also acts as a signal transducer for apoptosis, regulating the production of matrix metallo-proteins (MMP) and non-protein thiols. Additionally, eugenol induces p53 activation and the cleavage of proline-rich acidic protein (PRAP) in colon cancer cells.
Omega-3 fatty acids
Fatty acids consist of long-chain hydrocarbons with carbon numbers ranging from 10 to 30, and they are found in lipids. Eggs, seaweeds, microalgae, fish oil, marine fish, and algal oil all contain fatty acids, both saturated and unsaturated. Omega-3 polyunsaturated fatty acids, also known as PUFAs, have been the focus of extensive research in therapeutic and pathological settings. Both omega-3 polyunsaturated fatty acids and meals rich in nutraceuticals have been shown to impact human health. Omega-3 fatty acids have various therapeutic effects, including tumor development prevention and metastasis reduction. Even at high doses, omega-3 fatty acids can have an anti-catabolic effect on eicosanoid synthesis. In supplement form, essential fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) protect against colon and breast cancer in women.
This medication also reduces weight loss in cancer patients. Many clinical investigations have focused on a fish oil supplement with a high fat content and low carbohydrate count as a potential therapy for colon cancer. Omega-3 fatty acids found in fish oil supplements strengthen the immune system and accelerate the death of cancer cells.Omega-3 fatty acids’ anticancer properties are most effective in the early stages of colon cancer. By slowing the growth of colorectal polyps, omega-3 fatty acids may reduce the risk of developing early-stage colon cancer in individuals with a high risk.
Nutraceuticals effectively control the progression of colon cancer cell.
| Nutraceutical | Molecular Function | Secondary Metabolites | Source | Cancer Cell Line |
|---|---|---|---|---|
| Fisetin | Stimulation of NF-κB and reduction in cyclin D1 expression | Garlic crude extract | Allium sativum | HT-29 |
| Silbinin | Decreased cell growth | ———————— | ———————————————— | HT-29 |
| α-Tocopheral | Antiproliferation, oxidative phosphorylation | Salograriolide A | Centaurea ainetensis | HCT-116 |
| Curcumin (turmeric) | Triggered MAPK, Plsk/Akt | —————————— | —————————— | HT-29 |
| Fenugreek | Cytokines, redox reactions | —————————— | —————————— | HT-29 |
| Graph seed | Maintain the signaling associated epigenetics, oncogene expression | Avenanthramides | Oats | HCT-116 |
| Iron foods | Transferrin receptor (TfR1) | Centella asiatica crude extract | Whole plant | HCT-116, Cao-2 |
| Zerumbone | Upregulation of DR4 and DR5, MMP-9, Cdc family | Brachylaena ramiflora crude extract | Whole plant | HCT-116 |
| Rhizochalin | Caspase-3 and PRAP activation | —————————— | —————————— | HT-29 |
| Allicin | Control the overexpression of TNF-α family gene | Crude Lentinus edodes extract | Mushroom fruit bodies | HCT-116 |
| Fucoxanthin | Activate apoptosis | Oleuropein | Olive tree leaves | Caco-2, HT-29 |
| Garcinol | Suppression of tyrosine phosphorylation | —————————— | —————————— | HT-29/HCT-116 |
| Resveratrol | Triggered p53 gene for apoptosis | Grapes, wines | —————————— | HT-29/HCT-116 |
| Protein and peptides (milk) | Hypoproliferation of epithelium | Crude phenolics extract | Cichorium endlvia. L. | HCT-116 |
| Xanthohumol | Upregulation of caspase-3, -8, -9 | —————————— | Milk and milk-based products | HT-29 |
| Boswellic acid | Reduced cyclin D1 expression | Conjugated linoleic acid | Probiotic bacteria | HT-29/Colorectal cancer |
| β-Escin | Cell cycle progression arrest during the G1/S phase | —————————— | —————————— | HT-29 |
| Quercetin | Suppression of cyclin D1 expression | Procyanidins | Apple fruit | SW 480, SW 620 |
Information Source From Artical Colon cancer and colorectal cancer: Prevention and treatment by potential
natural products
Immunotherapy for Colon Cancer
Challenges and Emerging Strategies
Recent advancements in clinical oncology practice have brought immune system-altering treatments to the forefront, primarily due to the success of immune checkpoint inhibitors. While various cancers of the digestive tract have shown promising results with these treatments, colorectal cancers have not seen the same benefits. However, emerging data suggests that certain subsets of patients with hypermutated colorectal cancers may derive benefits from immune checkpoint inhibitors. Additionally, there are ongoing efforts to develop combination therapies that could potentially overcome the resistance observed in colorectal cancer. This article provides an overview of the molecular and immunologic landscape, along with a review of immunotherapeutic treatments currently being evaluated in colorectal cancer.
Molecular level in Immunotherapy for colon cancer
How has the understanding of colon cancer evolved in recent years?
Over the past two decades, researchers have made significant progress in understanding the step-wise pathogenesis of colorectal cancer, largely due to the identification of clear precursor lesions. These lesions provide insights into the progression of the disease. The Cancer Genome Atlas (TCGA) and international consensus groups have also been instrumental in establishing consensus definitions for colon cancer subtypes, which is crucial for advancing research and developing targeted therapies.
What are the key molecular alterations found in colon cancers?
A significant majority of colorectal cancers exhibit activation of the Wnt/β-catenin signaling pathway, often driven by the inactivation of the APC tumor suppressor gene. In metastatic CRC, mutations in RAS (specifically KRAS or NRAS) are seen in over 50% of patients, while BRAF mutations are found in about 5-10%. In addition, HER2 amplifications, present in 2-5% of CRC cases, are emerging as potential therapeutic targets
How does the genomic instability of coloncancer compare to other cancer types?
Colorectal cancers have a moderate level of genomic instability. They are positioned in the middle of the spectrum when compared to other cancer types in terms of average tumor mutation burden (TMB). However, this TMB can vary greatly across individual CRC cases, contributing to the heterogeneity observed in the disease.
Are there any other genetic mutations associated with hypermutated colon cancers?
Yes, in addition to MSI-H tumors, a small subset of hypermutated colorectal cancers carries polymerase mutations, particularly within the catalytic domains of DNA polymerase epsilon (POLE) or delta (POLD1). These mutations are significant because they result in an even higher mutation load, making these tumors strong candidates for immunotherapy, particularly immune checkpoint inhibitors.
What is the relevance of these molecular findings for immunotherapy in coloncancer?
The presence of hypermutation, particularly in MSI-H and POLE/POLD1-mutated colorectal cancers, makes these tumors more responsive to immunotherapy. The high mutation burden increases the likelihood of neoantigen formation, which can trigger an immune response. As a result, patients with these hypermutated cancers are more likely to benefit from immune checkpoint inhibitors, which have shown promising results in certain subsets of CRC, even though the broader CRC population has not experienced the same level of benefit from these treatments.
What is microsatellite instability (MSI-H), and how does it affect colon cancer?
Microsatellite instability (MSI-H) refers to tumors that have a significantly elevated mutational rate due to dysfunction of the mismatch repair (MMR) genes. MSI-H tumors represent a minority of CRC cases but are highly relevant in cancer subtyping and treatment, particularly in the context of immunotherapy. These tumors are most common in earlier stages of CRC—present in 22% of stage II, 12% of stage III, and only 3% of stage IV cancers. As the disease progresses, the frequency of MSI-H tumors decreases.
What does the future hold for Colon Cancer treatment based on these molecular insights?
The evolving understanding of the genetic landscape of colorectal cancer holds significant promise for the future of treatment. As researchers continue to refine cancer subtyping based on molecular alterations, more personalized and targeted therapies will become available. Immunotherapy, particularly for patients with hypermutated tumors, is a growing area of focus, and ongoing research into combination therapies aims to overcome resistance and improve outcomes for more CRC patients.
Colon Cancer Subtypes
What is the consensus molecular subtyping (CMS) of colorectal cancer?
Researchers have recently identified four consensus molecular subtypes (CMS) of colorectal cancer. This classification unifies previous criteria and relies on gene expression assays, much like the method used for determining breast cancer subtypes.
Is the CMS classification system used in routine patient care?
Currently, the CMS system is primarily used in research rather than for routine patient care. However, recent data suggest that these subtypes might be identified accurately using simple IHC-based assays, pending further validation.
What are the characteristics of the four CMS subtypes?
The four CMS subtypes are:
- CMS1 (MSI Immune, 14%): Characterized by hypermutation, microsatellite instability (MSI), and strong immune activation.
- CMS2 (Canonical, 37%): Epithelial tumors with chromosomal instability (CIN) and active WNT and MYC signaling.
- CMS3 (Metabolic, 13%): Epithelial tumors marked by metabolic dysregulation.
- CMS4 (Mesenchymal, 23%): Defined by TGF-β activation, stromal involvement, and angiogenesis.
How does the tumor microenvironment vary across CMS subtypes?
A recent analysis of independent colorectal cancer cohorts revealed differences in the tumor microenvironment for each subtype:
- CMS1: Overexpresses genes related to cytotoxic lymphocytes.
- CMS2 and CMS3: Show low inflammatory and immune signatures.
- CMS4: Characterized by angiogenic, inflammatory, and immunosuppressive signatures, with high fibroblast density and markers of lymphocytic and monocytic origin.
What are the implications of CMS subtypes for immunotherapy?
Different strategies are necessary for immunotherapy success based on the CMS subtype:
- CMS1 tumors: Likely to respond well to immune checkpoint inhibitors and therapies that reawaken the immune response.
- CMS4 tumors: Require targeting suppressive monocytoid cells and related cytokines, either alone or combined with immune checkpoint inhibitors.
- CMS2 and CMS3 tumors: Being ‘cold’ tumors, these may benefit from an immunogenic stimulus such as radiation, vaccines, or co-stimulatory compounds. These approaches are under development.
Additional Immunotherapy for colon cancer Approaches Underway
Combination with Chemotherapy
How can classic cytotoxic therapies affect the tumor microenvironment?
Researchers believe that classic cytotoxic therapies, such as chemotherapy and radiation, can induce immunogenic cell death (ICD). This process helps present tumor antigens and may stimulate an adaptive immune response.
Which therapies are thought to induce immunogenic cell death, and are their regimens well-defined?
Both chemotherapy and radiation therapy are thought to induce ICD. However, the ideal regimens for ICD induction are not yet well-defined. Drugs like 5-FU and oxaliplatin have been identified as having a positive impact.
Are there ongoing studies investigating immunogenic therapies for gastrointestinal cancers?
Yes, two studies are currently exploring the combination of FOLFOX with pembrolizumab for treating gastrointestinal and colon cancers.
What were the findings of the study combining atezolizumab and bevacizumab with or without chemotherapy?
In a study involving refractory patients, the combination of atezolizumab (anti-PD-L1 monoclonal antibody) and bevacizumab showed:
- 7% response rate (1 patient out of 14).
- 64% stable disease rate (9 patients), with two patients maintaining stability for 24 weeks.
What is the phase I trial of VEGF-TRAP, Aflibercept, and pembrolizumab investigating?
The trial (NCT02298959) is evaluating the combination of these agents with chemotherapy. It aims to determine how combining anti-angiogenic therapies with immune checkpoint inhibitors impacts cancer treatment.
What have researchers observed when combining FOLFOX, bevacizumab, and atezolizumab?
In a cohort of 30 patients:
- Among 23 treatment-naïve patients, 11 (48%) achieved a partial response.
- 20 out of 23 patients (87%) showed either a response or stable disease.
Tumor biopsies and blood samples indicated immune activation.
Are these results superior to chemotherapy alone?
It is unclear if the combination therapy results differ significantly from chemotherapy alone in this patient population. While response rates might be similar, the combination therapy could potentially enhance the durability of the response.
What additional data do we need to evaluate the effectiveness of combination therapies?
More comprehensive data on progression-free survival (PFS) and overall survival (OS) outcomes would provide deeper insights into the efficacy of these combination therapies.
Combinations with Radiotherapy
How are radiotherapy and thermal therapies linked to immunotherapy for colon cancer?
Many people believe that radiotherapy and thermal therapies have immunogenic properties and can lead to rare instances of abscopal responses, where distant tumors respond to localized therapy. Researchers have described this phenomenon for many years. However, recent preclinical models suggest that molecules like PD-1 may inhibit these responses.
Are there ongoing studies to test the immunotherapy for colon cancer effects of radiotherapy?
Yes, multiple studies are planned to test whether radiotherapy can facilitate successful immunological modulation. These trials aim to explore its potential to enhance immune responses.
What were the findings of the study combining liver-directed SBRT and PD-1 inhibitor AMP224 in metastatic CRC patients?
The study (NCT02298946) demonstrated safety in combining liver-directed stereotactic body radiation therapy (SBRT) with the PD-1 inhibitor, AMP224, for metastatic colorectal cancer (CRC) patients. However, no responses were observed.
What were the results of the study using radiofrequency ablation or radiotherapy with pembrolizumab in metastatic CRC?
The study (NCT02437071) showed interim results presented at ASCO 2016:
- Out of 22 patients receiving short-course radiotherapy, only one (4.5%) showed a response.
- No responses were observed in the ablation arm.
What is the current outlook for radiotherapy in immunotherapy for colon cancer?
Despite limited clinical responses so far, preclinical data are compelling. Multiple trials are planned to investigate various radiotherapy approaches. For example:
- NCT02888743: Evaluates radiotherapy combined with dual CTLA-4/PD-1 inhibitors.
- NCT02948348 and NCT02586610: Combine PD-1 inhibition with long-course chemoradiation in locally advanced rectal cancer.
When will the effectiveness of these approaches become clearer?
The value of combining radiotherapy with immunotherapy should become evident in the next few years as data from these ongoing trials emerge.
Additional Immunotherapy for colon cancer Combinations
What combinations are researchers studying to enhance immunotherapy for colon cancer?
Researchers are investigating agents that block suppressive immune factors, such as indoleamine 2,3-dioxygenase (IDO) and LAG-3, in phase I trials. In studies like NCT02178722, NCT02318277, NCT02327078, and NCT02460224, researchers are combining these with PD-1 or PD-L1 inhibitors. Additionally, direct immune stimulators like KIR and 4-1BB (CD137) are being studied in combination with PD-1 inhibition in trials such as NCT01714739 and NCT02179918.
What is the role of IDO in the tumor microenvironment?
So, IDO an enzyme breaking down non-dietary tryptophan, significantly impacts the tumor microenvironment by:
- Depleting tryptophan and increasing kynurenines, which induce T-cell apoptosis.
- Driving FoxP3 Treg differentiation.
- Promoting myeloid-derived suppressor cell (MDSC) accumulation and activation.
These effects create an immunosuppressive, tumor-permissive environment.
How does IDO impact immunotherapy outcomes for colon cancer?
In colorectal cancer patients, IDO weakens the immune response against tumors by reducing CD3 infiltrating T cells and leading to a worse prognosis..
Have there been clinical studies targeting IDO?
Yes, phase I studies using epacadostat (INCB024360) have shown:
- Successful inhibition of IDO1 activity.
- Reasonable tolerability.
However, single-agent activity has been limited.
What have studies combining epacadostat and pembrolizumab revealed?
In melanoma patients, a combination of epacadostat and pembrolizumab showed:
- An objective response rate of 58%.
- Complete responses in 26% of patients.
The combination was well-tolerated, but colorectal cancer-specific data is not yet available.
Are there ongoing trials involving IDO inhibitors in colon cancer?
Yes, IDO inhibitors are being evaluated in combination with PD-1 inhibitors in trials such as:
- NCT02327078.
- NCT02178722.
Additionally, a trial involving epigenetic modulation with azacitidine for lung cancer and MSS colorectal cancer is underway (NCT02959437).
Conclusion:
The American Society of Clinical Oncology (ASCO) declared Immunotherapy as the 2016 Clinical Cancer Advance of the Year. In 2017, they announced Immunotherapy 2.0 as the advance of the year. We are beginning to see success through the use of immunotherapy in colorectal cancer, particularly with PD-1 inhibition in MSI-H cancers. Targeting MSS cancers and non-hypermutated tumors is on the horizon. MEK and PD-L1 combinations are being tested, with multiple agents and combinations in development. Companies are shifting focus and investments toward immunotherapeutics. Additionally, adoptive cell therapy using tumor infiltrating lymphocytes (TILs) has shown remarkable success in colorectal cancer. Cancer immunotherapy strategies are moving ahead rapidly.
Information from Artical Immunotherapy for Colorectal Cancer
